What is Polyamide (Nylon)?
Polyamides or nylon are an important class of high-performance engineering thermoplastics due to their balanced properties. Polyamides contain repeating amide bonds, i.e. -CO-NH-. Nylon was discovered by Wallace Hume Carothers, a chemist hired by DuPont de Nemours in 1928 to lead an extensive research program to develop new polymeric materials. In 1935, he developed the formula known as PA 66:
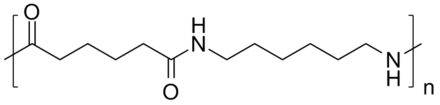
Polyamide 66
Polyamides are characterized by high temperature and electrical resistance. They also exhibit excellent chemical resistance thanks to their crystalline structure. They have excellent mechanical and barrier properties. In addition, these materials are highly flame retardant. When reinforced with glass fibers (short or long), the strength of their structure can compete with that of metals, which is why polyamides are often used as metal substitutes.
All polyamides tend to absorb moisture because of the amide chemical group. Moisture acts as a plasticizer in polyamides, reducing tensile modulus and increasing impact strength and flexibility. Moisture absorption also has a major effect on dimensional variations; this must be taken into account when designing parts.Polyamides are used in many applications, including automotive and transportation, electrical and electronics, consumer products, and many others.
Polyamide 6 (PA6) and Polyamide 66 (PA66)
Polyamide 6 (PA6) is also known as nylon 6 or polycaprolactam. It is one of the most widely used polyamides in the world. It is synthesized by ring-opening polymerization of caprolactam. The melting point of polyamide 6 is 223°C.
Polyamide 66 (PA66) or Nylon 66 is one of the most popular engineering thermoplastics and is mainly used as a replacement for metal in various applications. Nylon 66 is produced by polycondensation of hexamethylene diamine and adipic acid (two monomers with 6 carbon atoms each). The melting point of polyamide 66 is 255°C.
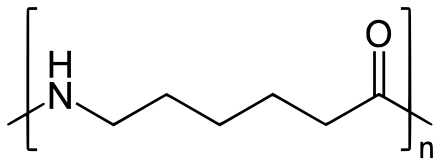
Polyamide 6
The problem of recycling PA6 and PA66
PA6 and PA66 are by far the most widely used polyamides in the world. Both polyamide 6 (PA6) and polyamide 66 (PA66) are used in many different markets and applications due to their excellent performance/cost ratio. Their key properties are listed below.
Although they have a similar set of properties, there are still slight differences. PA6 has slightly lower temperature resistance than PA66 and is also slightly less ductile. Compared to PA6, PA66 has the following characteristics:
- A slightly lower moisture absorption capacity
- Higher modulus
- Better wear resistance
- Better short-term heat resistance
These slight differences lead to a big problem in the polymer recycling industry: actually, PA6 and PA66 have different properties and processing requirements. Mixing them together for recycling purposes may result in a material with compromised properties, reducing its usability in applications that require specific performance characteristics.
The main way to recycle these polymers is mechanical recycling: in this process, the collected waste materials are shredded or granulated into small pieces. These pieces are then melted and extruded into pellets, which can be used to produce new plastic products.
NIRLAB Polymers supports the accurate differentiation between PA6 and PA66: our solution allows the user to skip this phase: in 5 seconds the user is able to distinguish between those two polymers without the need of melting and pelletting.
Results
As an example, the following are the results for the PA6 classifier, this classifier is combined in a more complex algorithm that is capable, by proper chaining of several machine learning models, to identify the polymer.
Every sample in this complex algorithm has been carefully checked by hand and so were the models: our strongness is flexibility in development which leads to tailor made solutions.
In the development several aspects have been taken into account such as colour, physical aspect, grade of washing, etc. in order to develop the most stable model possible.
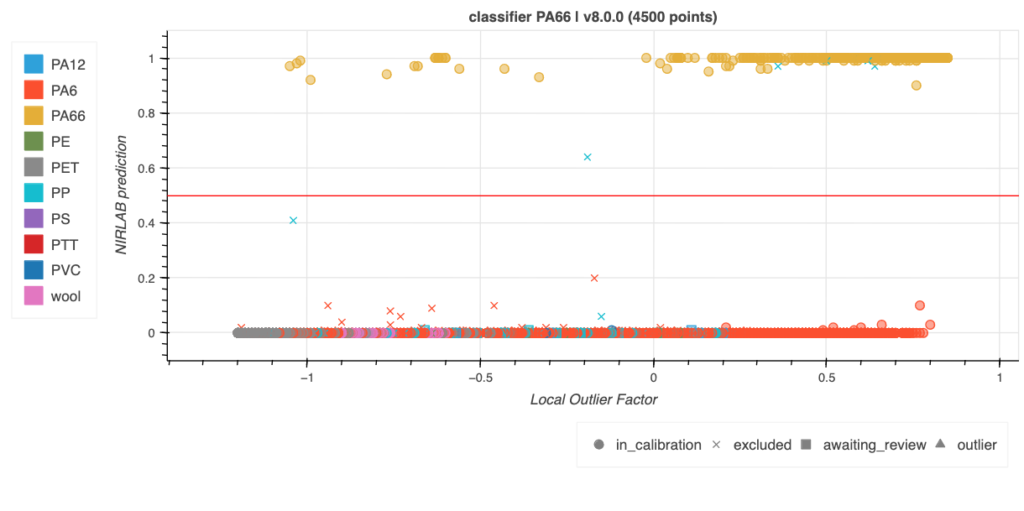
PA6 classifier results